Categories
- Pipe & Tube (18)
- Flange & Fitting (97)
- Fastener & Gasket (12)
- Valve & Pump (18)
- Base Material (11)
- Equipment (8)
- Application (30)
- Technical (110)
Wrought austenitic stainless steels refer to austenitic stainless steels which have been processed by one or a combination of two or more wrought processes such as forging, rolling, upsetting, bending, extruding, pressing and drawing. After the wrought process, austenitic stainless steels develop and attain a fiber like grain structure aligned in the principal direction of working. The fibers impart greater strength to the wrought products in the longitudinal direction of grain flow.
Chromium content of wrought austenitic stainless steels generally varies from 16% to 26%. They achieve stainless characteristics through the formation of an invisible and adherent chromium-rich oxide surface film. The oxide forms and heals itself in the presence of oxygen. The nickel content is up to about 35%, and manganese content may be up to 15%. Molybdenum, copper, silicon, aluminum, titanium, and niobium may be added to confer certain characteristics such as halide pitting resistance or oxidation resistance. Sulfur or selenium may be added to certain grades to improve machinability.
Austenitic stainless steels have a face-centered cubic (fcc) structure. This structure is attained through the liberal use of austenitizing elements such as nickel, manganese, and nitrogen. These steels are essentially nonmagnetic in the annealed condition and can be hardened only by cold working. They usually possess excellent cryogenic properties and good high-temperature strength. Moreover, they possess excellent ductility, formability, and toughness.
Wrought austenitic stainless steels can be subdivided into several categories: chromium-nickel alloys and chromium-manganese-nitrogen alloys. The whole austenitic stainless steel family can be viewed as originating from the standard austenitic stainless steel 304 (18Cr-8Ni or “18-8”). Type 316 is the modification by adding molybdenum for pitting resistance; More molybdenum addition forms Type 317; Type 309 and Type 310 are the modifications by adding more chromium and nickel for strength and oxidation resistance; Titanium, or niobium are added to Type 321/ Type 316Ti, or Type 347 respectively to stabilize the stainless steel against sensitization; More nickel, molybdenum, and nitrogen are added to attain super austenitic stainless steels or Ni-Cr-Fe alloys such as S31254, 904L, N08367, N08800, N08810, N08020, N08811, N08925, and N08926, which have better corrosion resistance even in high-temperature environments.
Wrought austenitic stainless steel compositions are based on a balance between alloy elements that promote ferrite formation and those that promote austenite formation. The prototype ferritizing element is chromium, but molybdenum, niobium, titanium, aluminum, tungsten, and vanadium also promote ferrite. The prototype austenitizing element is nickel, but carbon, nitrogen, and copper all promote transformation of ferrite to austenite at high temperatures. In addition, although manganese does not seem to promote transformation of ferrite to austenite at high temperatures, it clearly does tend to stabilize austenite with respect to transformation to martensite at low temperatures. Further, manganese promotes the solubility of nitrogen in steel (as does chromium), making possible a low-nickel family of austenitic stainless steels that are high in manganese and nitrogen.
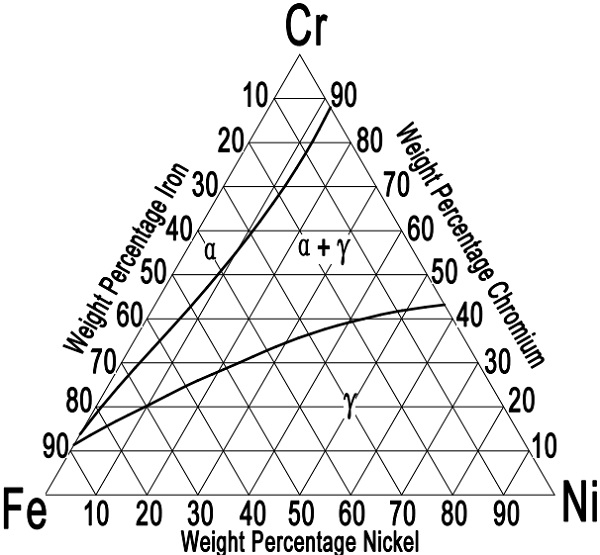
Figure-1: the iron-chromium-nickel ternary diagram for austenitic stainless steels at 1100°C [2012°F].
Austenitic stainless steels can be best described in terms of the iron-chromium-nickel ternary alloy system. The commercial alloys also contain a certain amount of carbon, silicon, manganese, sulfur, and so on. These elements might alter somewhat the phase balance, but by and large, the structure is determined by the three primary constituents iron, chromium, and nickel. Figure-1 shows the equilibrium iron-chromium-nickel phase diagram at 1100 °C (2012 °F). This is the temperature of maximum austenite stability and is used as the optimum annealing temperature for the 18-8 chromium-nickel steels. When comparing the ternary diagram with the binary iron-chromium diagram, it can be seen that the nickel addition extends the austenite phase field. The 18-8 steels fall in the gamma range, close to the iron-rich comer, beside the duplex phase field. Actually, above 1100°C [2012°F] some δ-ferrite will form. The phase diagram also indicates that as the chromium content increases above 18.0%, it also becomes necessary to raise the nickel content; otherwise, increasing amounts of ferrite will be formed.
